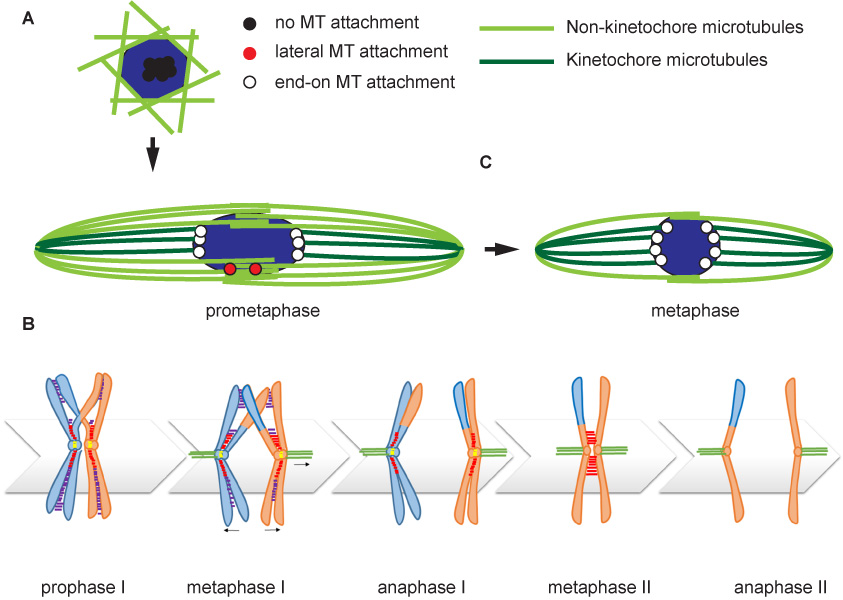
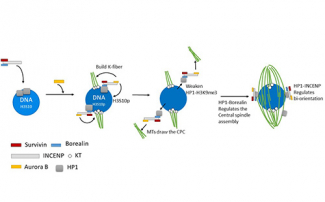
Characterize the relationship between CPC localization and bi-orientation
A key player in Drosophila meiosis is the four-protein chromosomal passenger complex (CPC). INCENP (Figure 2A) targets the complex to specific structures and recruits Aurora B, a kinase and the catalytic component of the CPC. In females expressing RNAi to Incenp or Aurora B, no meiotic spindle forms, showing that the CPC is required for chromosomes mediated spindle formation in oocytes.
To understand how the CPC is recruited to the chromosomes and promotes spindle assembly, we have generated mutants of Incenp that alter the localization pattern of the CPC. These mutated transgenes contain silent changes in the sequence that makes them RNAi resistant. In females expressing an RNAi resistant form of the wild-type gene, the meiotic spindle forms normally. When mutant Incenp transgenes that targeted the CPC to the centromeres or kinetochores are used, only kinetochore microtubules form, and there is no central spindle. This result suggests that targeting the CPC to the centromeres is sufficient to form a kinetochore but is not sufficient to promote bipolar spindle assembly. Several lines of evidence suggest that the key to CPC-mediated spindle assembly is heterochromatin protein 1 (HP1). First, in the absence of Aurora B, the rest of the CPC components (INCENP, Borealin, Survivin) colocalize with HP1 on the chromosomes. Second, mutants of Incenp or CPC subunit Borealin that disrupt HP1 interactions have meiotic spindle assembly defects. These experiments have shown that an interaction between HP1 and Borealin is required for the initial recruitment of the CPC to the chromosomes and the initiation of spindle assembly. Third, after spindle assembly, HP1 is mostly localized to the spindle. We propose a model for how HP1 orchestrates chromosome-mediated spindle assembly. HP1 is initially recruited to the heterochromatin by H3K9me2/3, where it recruits the CPC to promote spindle assembly. Aurora B then phosphorylates H3S10, which is known to disrupt HP1 localization. Therefore, the activity of Aurora B my cause the dissociation of HP1 from the chromatin, which may then force the CPC to move from the chromatin to the microtubules. Once on the spindle, the CPC promotes homologous chromosomes biorientation.
To identify the phosphatases that regulate Aurora B activity, we used a small molecule inhibitor of Aurora B, Binuclein 2 (BN2). When oocytes are treated with this drug, they lost their spindle microtubules. Thus, continuous Aurora B activity is required to preserve the spindle during meiosis I and spindle dynamics may be regulated by a phosphatase. When Aurora B was inhibited in PP2A RNAi oocytes, the spindle was maintained, demonstrating that PP2A antagonizes the Aurora B spindle maintenance function. We have further shown that the CPC negatively regulates KLP10A, a kinesin 13 that depolymerizes microtubules. Thus, we have found that PP2A opposes CPC functions, probably by dephosphorylating Aurora B spindle targets.
Page Components