Control of Growth and Morphogenesis
During development, organs grow to a characteristic size and shape. This is essential for normal organ function, and the symptoms of many congenital syndromes stem from defects in organ growth or morphogenesis. Moreover, dysregulation of growth control is associated with tumorigenesis. A detailed understanding of organ growth and morphogenesis will also be required to create functional organs from stem cells, which is a key goal of regenerative medicine. Yet how characteristic and reproducible organ size and shape are achieved remains poorly understood.
Key molecular insights into how growth is controlled have come from the identification and characterization in model systems of intercellular signaling pathways that are required for the normal control of organ growth. Many of these pathways are highly conserved among different phyla. We are engaged in projects whose long-term goals are to define relationships between patterning and growth in developing and regenerating organs and to determine how these patterning inputs are integrated with other factors that influence organ growth, such as nutrition and mechanical stress. Much of our research takes advantage of the powerful genetic, molecular, and cellular techniques available in Drosophila melanogaster, which facilitate both gene discovery and the analysis of gene function. We also use cultured mammalian cell models and organoids.
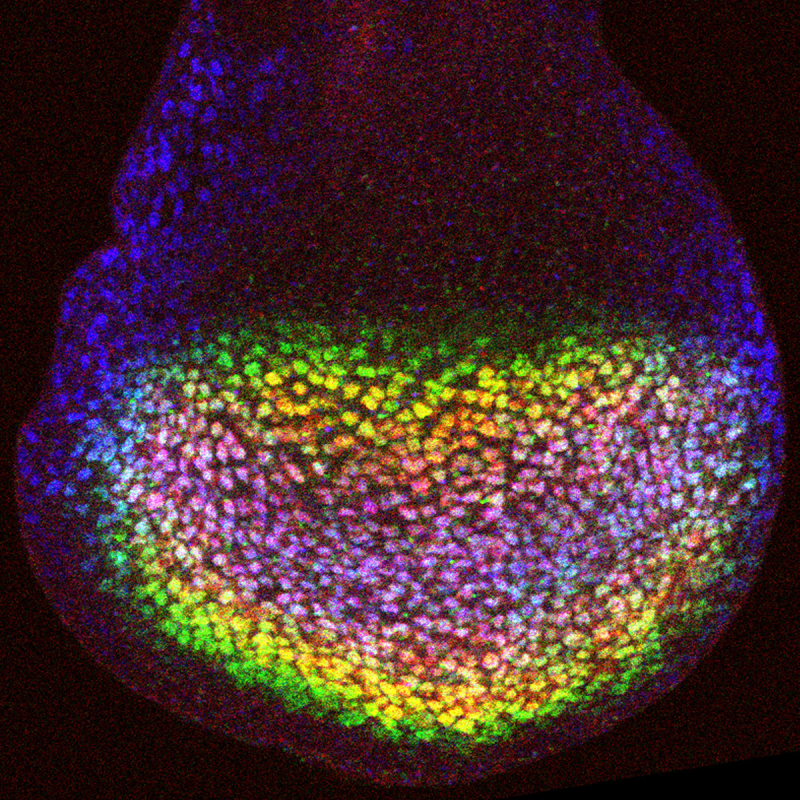
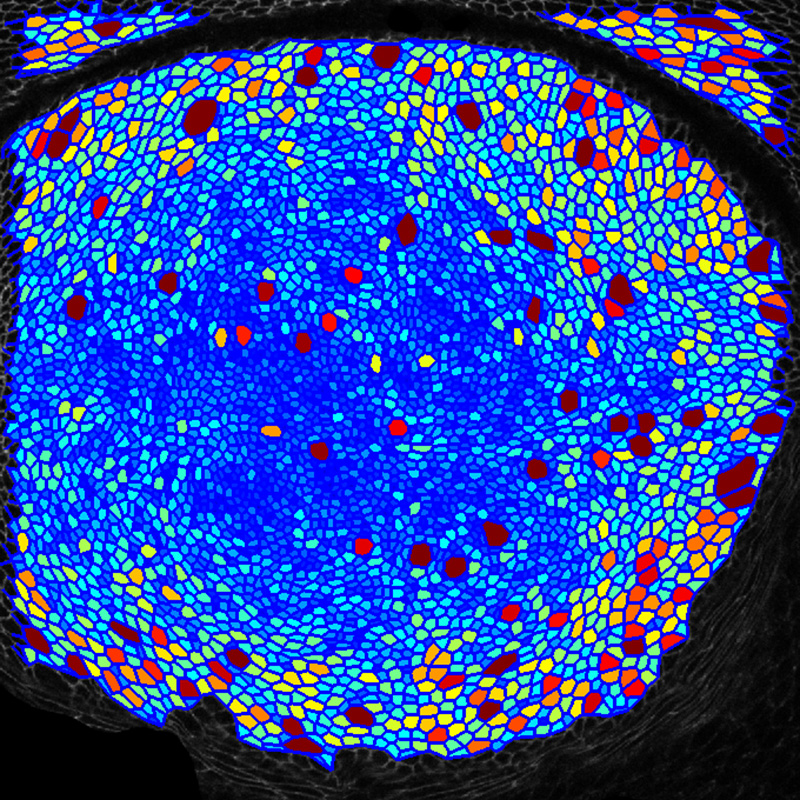
One major area of research has involved investigations of the Hippo signaling network, which has emerged over the past decade as one of the most important growth regulatory pathways in animals. We study its regulation, molecular mechanisms of signal transduction, and its roles in different developmental and physiological contexts. A recent focus of our efforts on Hippo signaling have involved investigations of how mechanical forces experienced by cells influence Hippo signaling, and thereby organ growth. Observations that mechanical stress can influence cell proliferation had been made as early as the 1960s. However, the molecular mechanisms responsible were unknown. We identified the first biomechanical pathway that could link cytoskeletal tension to Hippo signaling by discovering that the localization and activity of the Drosophila Ajuba LIM protein (Jub), and the Warts kinase, are modulated by cytoskeletal tension, providing a direct link between mechanical tension and organ growth. We have since demonstrated that this mechanism contributes to feedback regulation of growth in compressed cells, and that it contributes to density-dependent regulation of cell proliferation in developing Drosophila wings. We have also characterized links between mechanical forces and Hippo signaling in mammalian cells, and discovered both conservation of this Jub biomechanical pathway and identified a role for it in cell density-dependent regulation of mammalian Hippo signaling, including contact-inhibition of cell proliferation. Our studies have provided a molecular understanding of how tissue mechanics can influence Hippo signaling, while also emphasizing that there are multiple mechanisms by which mechanical forces regulate this pathway.
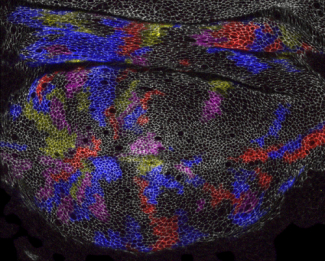
We also investigate how tissue patterning and mechanics influence morphogenesis. As one simple model, we have combined genetic analysis, live imaging, and computation image analysis to investigate cellular and molecular mechanism that govern wing shape in Drosophila, with a particular focus on the contributions of tissue mechanics.
Page Components