Severinov: Discovery of 30-year mystery molecular machine behind microcin B17
The potent natural antibiotic, microcin B17, kills harmful E. coli bacteria. Microbial resistance to antibiotics- due to their overuse and misuse—is one of the biggest threats facing humanity, and there's an urgent need to find new drugs. Natural antibiotics that evolved over eons represent an attractive option to overcome resistance.
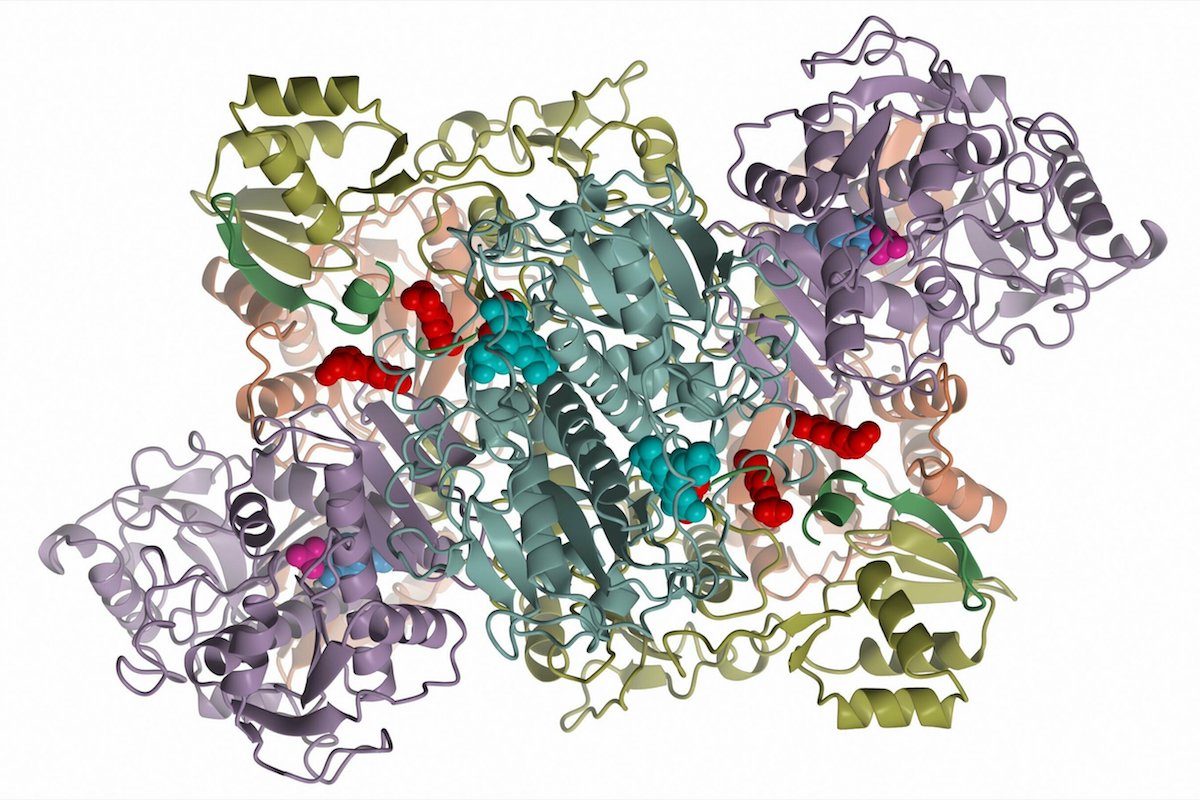
Scientists have known about microcin B17 and its unusual chemical structure for decades, but they did not understand the molecular machinery that makes it until now. In a jount study, scientists at Rutgers and universities in Russia, Poland and England found that an enzyme called McbBCD triggers two chemical reactions that produce several chemical “cycles” required for antibacterial activity, according to senior author Konstantin Severinov, a principal investigator at the Waksman Institute of Microbiology and professor of molecular biology and biochemistry at Rutgers University-New Brunswick.
The discovery has the potential to provide the tools to design new antibiotics, anticancer drugs and other therapeutics.
"Our research allows rational design of new peptide compounds that could become treatments ranging from antimicrobials to anticancer drugs," Severinov said. “There may be a trove of new antibiotics that could be made from peptides, using enzyme machines like McbBCD as a production tool.”