Expression of recombinant proteins in chloroplasts
The laboratory has a long tradition in the expression of recombinant proteins in chloroplasts. Most recent is development of dicistronic operons as a novel marker system for chloroplast engineering that can be used as building blocks for plant synthetic biology. The identification of transplastomic clones is based on selection for antibiotic resistance encoded in the first open reading frame (ORF) and accumulation of the reporter gene product in tobacco chloroplasts encoded in the second ORF. The antibiotic resistance gene may encode spectinomycin or kanamycin resistance based on the expression of aadA or neo genes, respectively. The reporter gene used in the study is the green fluorescent protein (GFP). The mRNA level depends on the 5’ UTR of the first ORF. The protein output depends on the strengths of the ribosome binding, and is proportional with the level of translatable mRNA. Because the dicistronic mRNA is not processed, we could show that protein output from the second ORF is independent from the first ORF (Figure 1). High-level GFP accumulation from the second ORF facilitates identification of transplastomic events under UV light. Expression of multiple proteins from an unprocessed mRNA is an experimental design that enables predictable protein output from polycistronic mRNAs, expanding the toolkit of plant synthetic biology. These data have been described in a recent publication (Yu et al., 2020 Plant Journal doi: 10.1111/tpj.14864).
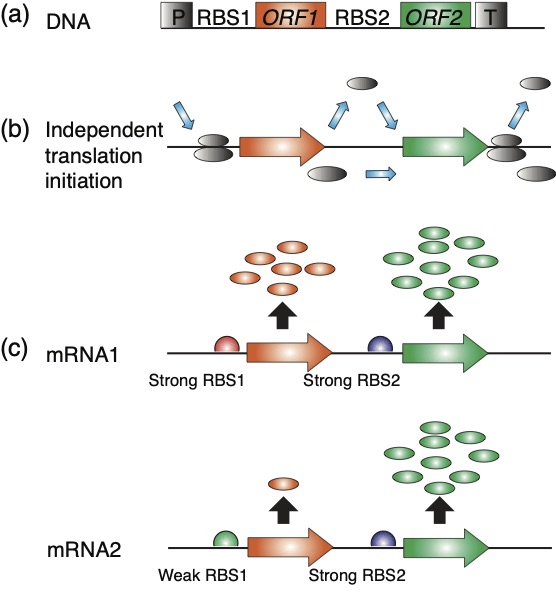
(a) Schematic map of dicistronic operons. ORF1 encodes a marker gene, the second ORF gfp. The operon has one promoter (P) and one 3’-UTR (T) for the stabilization of the mRNA.
(b) Translation initiates independently from the first and second ORFs. Shown are the small and large ribosomal subunits entering the mRNA at the ribosome entry site, and dissociating when translation of the 1st ORF is completed. Small ribosomal subunits initiate translation independently of the 2nd ORF.
(c) Protein accumulation from the first ORF has no significant impact on protein accumulation from the 2nd ORF. Protein output from the 1st ORF can be high (pMRR20) or low (pMRR21), the protein output from the 2nd ORF is always high