Dong Lab
Research Overview
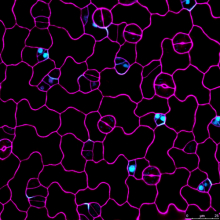
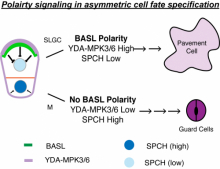
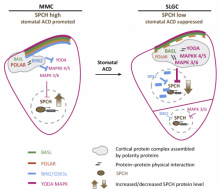
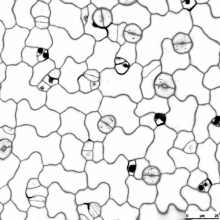
Cell polarity, in both animals and plants, is of paramount importance for many developmental and physiological processes. In the future, my lab will continue to use Arabidopsis as a model system, by studying BASL (Breaking of Asymmetry in the Stomatal Lineage) and other newly identified factors, to investigate how proteins become polarly localized, how polarity proteins are involved in establishment of cellular asymmetry, and how cell polarity is instructive of cell fate and differentiation in plants.
Contact Information
Waksman Institute
190 Frelinghuysen Road
Dong Lab, Office 1012A
Piscataway, NJ 08854
United States
Selected Publications
Complete list of publications: [Google Scholar] [Pubmed]
Current Lab Members
Principal Investigator
-
Dr. Juan Dong is a Professor of Plant Biology. Before joining Rutgers, Juan was a post-doctoral researcher in Dominique Bergmann’s laboratory in the Department of Biology at Stanford University. Juan received her Ph.D. in Plant Biology with Dr. Elizabeth Lord at the University of California at Riverside.
Dr. Dong's research has focused on mechanisms of asymmetric cell division, a fundamental problem in morphogenesis, which is the biological process that causes an organism to develop its shape. She used a genetic approach to identify a mutant phenotype of the plant protein called BASL (Breaking of Asymmetry in the Stomatal Lineage). The corresponding gene controls asymmetric division in Arabidopsis, a small flowering plant in the mustard family.
Postdoctoral Researchers
Alumni
Former Research Associates
- Ying Zhang
Former Postdoctoral Fellows
- Xueyi Xue
Former Graduate Students
- Lu Wang
- Chao Bian
- Wanchen Shao
Former Undergraduate Students
- Gaurav Pathak
- Kavya Mehndiratta