Dismukes Lab
Research Overview
To mitigate many of the potentially deleterious environmental and agricultural consequences associated with current landbased-biofuel feedstocks, we advocate the use of biofuels derived from aquatic microbial oxygenic photoautotrophs (AMOPs), more commonly known as algae, cyanobacteria and diatoms. Our research on AMOPs addresses:
-
Demonstrated productivity in mass culturing and future potential as biomass energy crops
-
Use as cell factories for production of gaseous fuels (H2, CH4)
-
Fundamental photosynthetic physiology and mechanisms
-
Genetic transformants for understanding mechanisms and improving fuel production.
Announcements
-
-Congratulations Hiba Shaqra!
Please join me in congratulating Hiba Shaqra on her nomination by Rutgers University for a Goldwater fellowship. This is considered the most prestigious undergraduate fellowship in the natural sciences, mathematics and engineering in America.
Hiba is a junior majoring in Molecular Biology and Biochemistry | Arabic, Chemistry, and Mathematics minors. She has been working closely with Apostolos Zournas for the last two years in the Waksman Institute. Pat on the back to Apostolos for awesome mentoring!
-
-Rutgers University inventors Charles Dismukes, Anders Laursen, Karin Calvinho, and Martha Greenblatt were recognized for an Edison Patent Award in the Environmental category
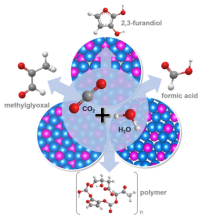
Distinguished Professor Charles Dismukes from Rutgers University with co-inventors, Anders Laursen, Karin Calvinho, and Martha Greenblatt have been honored with the Edison Patent Award in the Environmental category for their invention, “Nickel Phosphide Catalysts for Direct Electrochemical CO₂ Reduction to Hydrocarbons” (U.S. Patent 10,676,833).
-
Catalysis Research
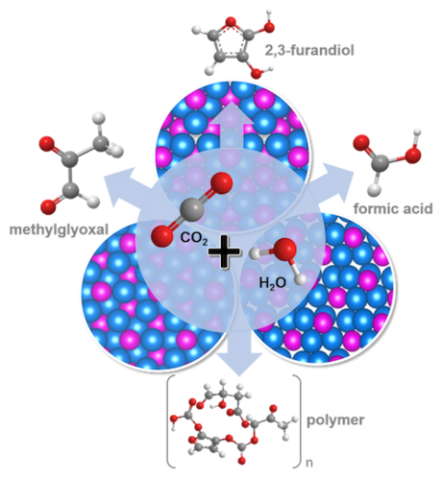
We develop selective and robust catalysts that electrochemically convert carbon dioxide (CO2) into sustainable chemical feedstocks and could ultimately be coupled to the recycling of environmental CO2. The catalysts employed are transition metal phosphides and their doped derivatives that form distinct crystalline structure types, enabling selection of chemical reactivity towards desired products including high molecular weight solid polymers. Selecting the catalyst’s elemental composition and crystal structure allows for tuning of the chemical, physical, and… Read More
Contact Information
Chemistry & Chemical Biology
610 Taylor Road
Wright Rieman Labs 102
Piscataway, NJ 08854
United States
Selected Publications
Complete list of publications: [Google Scholar] [Pubmed]
'Birth defects’ of photosystem II make it highly susceptible to photodamage during chloroplast biogenesis.
Shevela, D., G. Ananyev, A.K. Vatland, J. Arnold, F. Mamedov, L. Eichacker, G.C. Dismukes and J. Messinger, 'Birth defects’ of photosystem II make it highly susceptible to photodamage during chloroplast biogenesis. Physiol. Plant., 2019. https://doi.org/10.1111/ppl.12932
Understanding Water and Gas Transport in Algal-Fungal Symbionts: Integrating Laboratory Experiments and Numerical Modeling of Lichens
Potkay, A., M.-C.t. Veldhuis, G. Ananyev, G.C. Dismukes and Y. Fan, Understanding Water and Gas Transport in Algal-Fungal Symbionts: Integrating Laboratory Experiments and Numerical Modeling of Lichens. in preparation, 2019.
Crossing the Thauer limit: rewiring cyanobacterial metabolism to maximize fermentative H2 production
Kumaraswamy, G.K., A. Krishnan, G. Ananyev, D.A. Bryant and G.C. Dismukes, Crossing the Thauer limit: rewiring cyanobacterial metabolism to maximize fermentative H2 production. Energy & Environmental Science, 2019. Advance article(c8ee03606c): p. c8ee03606c.
Fluorescence induction technique for studies of photosynthetic electron transport across multiple time domains: Application to CO2-fixation rates and fluxes.
Ananyev, G., A. Zournas, C. Gates, M.-c.t. Veldhuis and G.C. Dismukes, Fluorescence induction technique for studies of photosynthetic electron transport across multiple time domains: Application to CO2-fixation rates and fluxes. in preparation, 2019.
The Catalytic Cycle of Water Oxidation in Crystallized Photosystem II Complexes: Performance and Requirements for Formation of Intermediates.
Ananyev, G., S. Roy-Chowdhury, C. Gates, P. Fromme and G.C. Dismukes, The Catalytic Cycle of Water Oxidation in Crystallized Photosystem II Complexes: Performance and Requirements for Formation of Intermediates. ACS Catalysis, 2019. 9(2): p. 1396-1407.
Desiccation tolerant lichens facilitate in vivo H/D isotope effect measurements in oxygenic photosynthesis.
Vinyard, D.J., G.M. Ananyev and G.C. Dismukes, Desiccation tolerant lichens facilitate in vivo H/D isotope effect measurements in oxygenic photosynthesis. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 2018. 1859(10): p. 1039-1044.
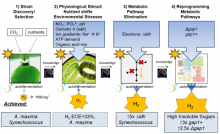
Rerouting of Metabolism into Desired Cellular Products by Nutrient Stress: Fluxes Reveal the Selected Pathways in Cyanobacterial Photosynthesis.
Qian, X., Y. Zhang, D.S. Lun and G.C. Dismukes, Rerouting of Metabolism into Desired Cellular Products by Nutrient Stress: Fluxes Reveal the Selected Pathways in Cyanobacterial Photosynthesis. ACS Synthetic Biology, 2018. 7(5): p. 1465-1476.
Rewiring of cyanobacterial metabolism for hydrogen production: Synthetic biology approaches and challenges. Synthetic Biology of Cyanobacteria
Krishnan, A., X. Qian, G. Ananyev, D.S. Lun and G.C. Dismukes, Rewiring of cyanobacterial metabolism for hydrogen production: Synthetic biology approaches and challenges. Synthetic Biology of Cyanobacteria, in Adv Exp Med Biol. , W.Z.a.X. Song, Editor. 2018, Springer: Singapore. p. 171-213.
Resolving Ambiguous Protonation and Oxidation States in the Oxygen Evolving Complex of Photosystem II.
Chen, H., G.C. Dismukes and D.A. Case, Resolving Ambiguous Protonation and Oxidation States in the Oxygen Evolving Complex of Photosystem II. The Journal of Physical Chemistry B, 2018. 122(37): p. 8654-8664.
Reconciling Structural and Spectroscopic Fingerprints of the Oxygen-Evolving Complex of Photosystem II: A Computational Study of the S2 State
Chen, H., D.A. Case and G.C. Dismukes, Reconciling Structural and Spectroscopic Fingerprints of the Oxygen-Evolving Complex of Photosystem II: A Computational Study of the S2 State. The Journal of Physical Chemistry B, 2018. 122(50): p. 11868-11882.
Nickel phosphides catalyze the electrochemical CO2 reduction to C3 and C4 products at low overpotential
Calvinho, K.U.D., A.B. Laursen, K.M.K. Yap, T.A. Goetjen, S. Hwang, N. Murali, B. Mejia-Sosa, A. Lubarski, K.M. Teeluck, E. Garfunkel, M. Greenblatt and G.C. Dismukes, Nickel phosphides catalyze the electrochemical CO2 reduction to C3 and C4 products at low overpotential. Energy and Environmental Science, 2018. 11(9): p. 2550-2559.
The multiplicity of roles for (bi)carbonate in photosystem II operation in the hypercarbonate-requiring cyanobacterium Arthrospira maxima
Ananyev, G., C. Gates and G.C. Dismukes, The multiplicity of roles for (bi)carbonate in photosystem II operation in the hypercarbonate-requiring cyanobacterium Arthrospira maxima. Photosynthetica, 2018. 56(1): p. 217-228. doi.org/10.1007/s11099-018-0781-0
Climbing the volcano of electrocatalytic activity while avoiding catalyst corrosion: Ni3P a hydrogen evolution electrocatalyst stable in both acid and alkali
A. B. Laursen, R. B. Wexler, M. J. Whitaker, E. Izett, R. Rucker, H. Wang, J. Li, M. Greenblatt, A. M. Rappe and G.C. Dismukes, Climbing the volcano of electrocatalytic activity while avoiding catalyst corrosion: Ni3P a hydrogen evolution electrocatalyst stable in both acid and alkali. ACS Catalysis, 2018(8): p. 4408−4419. https://doi.org/10.1021/acscatal.7b04466
Flux balance analysis of photoautotrophic metabolism: Uncovering new biological details of subsystems involved in cyanobacterial photosynthesis
Qian, X., M.K. Kim, G.K. Kumaraswamy, A. Agarwal, D.S. Lun and G.C. Dismukes, Flux balance analysis of photoautotrophic metabolism: Uncovering new biological details of subsystems involved in cyanobacterial photosynthesis. Biochim Biophys Acta, 2017. 1858(4): p. 276-287. https://doi.org/10.1016/j.bbabio.2016.12.007
Biogenesis and Assembly of the CaMn4O5 Core of Photosynthetic Water Oxidases and Inorganic Mutants, in Metalloprotein Active Site Assembly
Gates, C., G. Ananyev and C.C. Dismukes, Biogenesis and Assembly of the CaMn4O5 Core of Photosynthetic Water Oxidases and Inorganic Mutants, in Metalloprotein Active Site Assembly, M.K. Johnson and R.A. Scott, Editors. 2017, John Wiley & Sons, Ltd, : Chichester, West Sussex PO19 8SQ, UK. p. 1-15.
The Oxygen quantum yield in diverse algae and cyanobacteria is controlled by partitioning of flux between linear and cyclic electron flow within photosystem II
Ananyev, G., C. Gates and G. Dismukes, The Oxygen quantum yield in diverse algae and cyanobacteria is controlled by partitioning of flux between linear and cyclic electron flow within photosystem II. . Biochimica et Biophysica Acta (BBA)-Bioenergetics, 2017. 1857 (9): p. 1380-1391. doi: 10.1016/j.bbabio.2016.04.056
Natural and Synthetic Variants of the Tricarboxylic Acid Cycle in Cyanobacteria: Introduction of the GABA Shunt into Synechococcus sp. PCC 7002
Zhang, S., X. Qian, S. Chang, G.C. Dismukes and D.A. Bryant, Natural and Synthetic Variants of the Tricarboxylic Acid Cycle in Cyanobacteria: Introduction of the GABA Shunt into Synechococcus sp. PCC 7002. Front Microbiol, 2016. 7: p. 1972. doi: 10.3389/fmicb.2016.01972
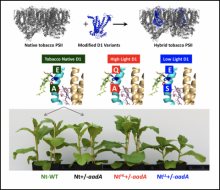
Natural isoforms of the Photosystem II D1 subunit differ in photoassembly efficiency of the water-oxidizing complex
Vinyard, D.J., J.S. Sun, J. Gimpel, G.M. Ananyev, S.P. Mayfield and G. Charles Dismukes, Natural isoforms of the Photosystem II D1 subunit differ in photoassembly efficiency of the water-oxidizing complex. Photosynth Res, 2016. 128(2): p. 141-50. doi: 10.1007/s11120-015-0208-8
Coordination Geometry and Oxidation State Requirements of Corner Sharing MnO6 Octahedra for Water Oxidation Catalysis: An Investigation of Manganite (γ-MnOOH)
Smith, P.F., B.J. Deibert, S. Kaushik, G. Gardner, S. Hwang, H. Wang, J.F. Al-Sharab, E. Garfunkel, L. Fabris, J. Li and G.C. Dismukes, Coordination Geometry and Oxidation State Requirements of Corner Sharing MnO6 Octahedra for Water Oxidation Catalysis: An Investigation of Manganite (γ-MnOOH). ACS Catalysis, 2016. 6(3): p. 2089–2099. https://doi.org/10.1021/acscatal.6b00099
Inactivation of nitrate reductase alters metabolic branching of carbohydrate fermentation in the cyanobacterium Synechococcus sp. strain PCC 7002.
Qian, X., G.K. Kumaraswamy, S. Zhang, C. Gates, G.M. Ananyev, D.A. Bryant and G.C. Dismukes, Inactivation of nitrate reductase alters metabolic branching of carbohydrate fermentation in the cyanobacterium Synechococcus sp. strain PCC 7002. Biotechnol Bioeng, 2016. 113(5): p. 979-88. doi: 10.1002/bit.25862
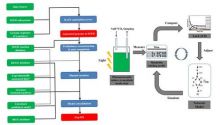
Optimizing “Artificial Leaf” Photoanode-Photocathode-Catalyst Interface Systems for Solar Water Splitting
Porter, S.H., S. Hwang, V. Amarasinghe, E. Taghaddos, V. Manichev, M. Li, G. Gardner, A. Safari, E. Garfunkel, M. Greenblatt and G.C. Dismukes, Optimizing “Artificial Leaf” Photoanode-Photocathode-Catalyst Interface Systems for Solar Water Splitting. ECS Transactions, 2016. 72(37): p. 1-19. https://doi.org/10.1149/07237.0001ecst
Consequences of ccmR deletion on respiration, fermentation and H2 metabolism in cyanobacterium Synechococcus sp. PCC 7002
Krishnan, A., S. Zhang, Y. Liu, K.A. Tadmori, D.A. Bryant and C.G. Dismukes, Consequences of ccmR deletion on respiration, fermentation and H2 metabolism in cyanobacterium Synechococcus sp. PCC 7002. Biotechnol Bioeng, 2016. 113(7): p. 1448-59. doi: 10.1002/bit.25913
Thin Film Catalysts: Ni5P4 (Cathodic) and LiCoO2 (Anodic) for Electrolysis of Water
Hwang, S., S.H. Porter, G. Gardner, A.B. Laursen, H. Wang, M. Li, V. Amarasinghe, E. Taghaddos, A. Safari, E. Garfunkel, M. Greenblatt and G.C. Dismukes, Thin Film Catalysts: Ni5P4 (Cathodic) and LiCoO2 (Anodic) for Electrolysis of Water. ECS Transactions, 2016. 72(23): p. 31-51. https://doi.org/10.1149/07223.0031ecst
The strontium inorganic mutant of the water oxidizing center (CaMn4O5) of PSII improves WOC efficiency but slows electron flux through the terminal acceptors
Gates, C., G. Ananyev and G.C. Dismukes, The strontium inorganic mutant of the water oxidizing center (CaMn4O5) of PSII improves WOC efficiency but slows electron flux through the terminal acceptors. Biochim Biophys Acta, 2016. 1857(9): p. 1550-1560. doi: 10.1016/j.bbabio.2016.06.004
Structural basis for differing electrocatalytic water oxidation by the cubic, layered and spinel forms of lithium cobalt oxides
Gardner, G., J. Al-Sharab, N. Danilovic, Y.B. Go, K. Ayer, M. Greenblatt and G.C. Dismukes, Structural basis for differing electrocatalytic water oxidation by the cubic, layered and spinel forms of lithium cobalt oxides. Energy Environ. Sci., 2016. 9: p. 184--192. DOI: 10.1039/c5ee02195b
The unexpected extremophile: Tolerance to fluctuating salinity in the green alga Picochlorum
Foflonker, F., G. Ananyev, H. Qiu, A. Morrison, B. Palenik, G.C. Dismukes and D. Bhattacharya, The unexpected extremophile: Tolerance to fluctuating salinity in the green alga Picochlorum. Algal Research-Biomass Biofuels and Bioproducts, 2016. 16: p. 465-472. doi.org/10.1016/j.algal.2016.04.003
X-ray Emission Spectroscopy of Mn Coordination Complexes Toward Interpreting the Electronic Structure of the Oxygen-Evolving Complex of Photosystem II
Davis, K.M., M.C. Palenik, L.F. Yan, P.F. Smith, G.T. Seidler, G.C. Dismukes and Y.N. Pushkar, X-ray Emission Spectroscopy of Mn Coordination Complexes Toward Interpreting the Electronic Structure of the Oxygen-Evolving Complex of Photosystem II. Journal of Physical Chemistry C, 2016. 120(6): p. 3326-3333.
Photosystem II-cyclic electron flow powers exceptional photoprotection and record growth in the microalga Chlorella ohadii
Ananyev, G., C. Gates, A. Kaplan and G.C. Dismukes, Photosystem II-cyclic electron flow powers exceptional photoprotection and record growth in the microalga Chlorella ohadii. Biochim Biophys Acta, 2016. 1858(11): p. 873-883. doi: 10.1016/j.bbabio.2017.07.001
Selective CO 2 Reduction to C 3 and C 4 Oxyhydrocarbons on Nickel Phosphides at Overpotentials as Low as 10 MV.
Calvinho, Karin U. D., Anders B. Laursen, Kyra M. K. Yap, Timothy A. Goetjen, Shinjae Hwang, Bryan Mejia-Sosa, Alexander Lubarski, et al. 2018. “Selective CO 2 Reduction to C 3 and C 4 Oxyhydrocarbons on Nickel Phosphides at Overpotentials as Low as 10 MV.” Energy & Environmental Science. The Royal Society of Chemistry. doi:10.1039/C8EE00936H.
Current Lab Members
Principal Investigator
-
G. Charles Dismukes is a Distinguished Professor at Rutgers University on the faculties of the Chemistry & Chemical Biology Department and the Waksman Institute of Microbiology. He is a member of the executive committee of the Institute for Advanced Materials and Device Nanotechnology (IAMDN), the Rutgers Energy Institute (REI), and the graduate training faculty in Microbiology and Biochemistry.
The Dismukes research group investigates the science of catalysis particularly photo- and electro-catalysis, using an integrated theoretical-experimental approach that incorporates knowledge from biology, chemistry and physics to understand the fundamental principles and translate that knowledge into proof of principle devices and solutions. They study systems extending from natural photosynthetic organisms and isolated enzymes to artificial photosynthetic constructs and solar fuel cells.
Topics include water splitting, carbon dioxide capture and reduction to chemicals, carbon metabolism in phototrophs, dinitrogen capture and reduction.
Alumni
Former Research Associates
- Gennady Ananyev
Former Graduate Students
- Yuan Zhang